Clinical and immunological features of opisthorchiasis
- Authors: Sabitov AU1, Soldatov DA1, Khamanova Y.B1
-
Affiliations:
- Ural State Medical University
- Issue: Vol 102, No 5 (2021)
- Pages: 626-635
- Section: Theoretical and clinical medicine
- Submitted: 19.04.2021
- Accepted: 16.08.2021
- Published: 13.10.2021
- URL: https://kazanmedjournal.ru/kazanmedj/article/view/65212
- DOI: https://doi.org/10.17816/KMJ2021-626
- ID: 65212
Cite item
Abstract
Aim. To assess the clinical and immunological features in patients with chronic opisthorchiasis, depending on the duration of the infection.
Methods. The first group consisted of 19 patients with the duration of the infection up to 1 year, the second group consisted of 21 patients with the duration of the infection between 1 and 5 years, the third group was formed of 23 patients with the duration of the disease more than 5 years, the control group — 20 healthy individuals. Immunological research was carried out at the Clinical Diagnostic Center. Statistical processing was performed using Microsoft Excel 2010 and Statistica 6.0 software. The statistical significance of differences was determined by using the Mann–Whitney test (U-test) at the level of significance of p <0.05. The correlations were assessed by calculating Spearman's rank correlation coefficients.Results Clinical features of chronic opisthorchiasis were revealed in the disease duration groups of up to 1 year, from 1 year to 5 years, more than 5 years: the subclinical course was most common in the group of up to 1 year; cholangiohepatitis prevailed in the group of between 1 to 5 years, allergic skin syndrome, cholangiocholecystitis and pancreatitis dominated in the group of more than 5 years. The immune response in chronic opisthorchiasis was characterized by: up to 1 year — lymphocytosis, increased levels of immunoglobulins M (IgM) and circulating immune complexes (CIC), a decrease in the number of T-lymphocytes (CD3+), as well as an increase in bactericidal activity of leukocytes (BAL); between 1 and 5 years — monocytosis, increased levels of immunoglobulins M, immunoglobulins G and circulating immune complexes, a decrease in T-cytotoxic lymphocytes (CD8+) and nitro blue tetrazolium (NBT test), as well as an increase in NK cells and phagocytic activity of monocytes, more than 5 years — eosinophilia.
Conclusion. Common features of rearrangement of the immune system in opisthorchiasis: inflammatory changes in the hemogram, activation of humoral immunity with parallel suppression of the cellular component of the immune system, and increased phagocytosis.
Full Text
Актуальность. Причиной хронизации патологического процесса при описторхозе становятся длительное паразитирование гельминтов в органах гепатобилиарной системы и поджелудочной железе, токсическое влияние на органы желудочно-кишечного тракта, приводящее к развитию хронического холецистита, холангита, гепатита, панкреатита, гастрита, дуоденита, язвенной болезни желудка и двенадцатиперстной кишки [1–3].
Возникновение активного гепатита, особенно в случаях суперинвазии и реинвазии, ряд авторов связывают с иммунным воспалением [4]. У части больных развивается панкреатит, отличающийся волнообразным течением с частой сменой периодов обострений и ремиссий, и редко разворачивается прогрессирующее течение. Описторхоз нередко сопровождается поражением кожи, что бывает следствием аллергической реакции организма на внедрение паразита [5]. Аллергический кожный синдром при хроническом описторхозе может проявляться зудом, эритемой, крапивницей, в тяжёлых случаях отёком Квинке, в периферической крови — эозинофилией [5, 6].
В прикладном аспекте наиболее актуальны вопросы о патофизиологических механизмах функционирования иммунной системы в условиях хронической инвазии [7]. В ряде работ показано, что в активной стадии заболевания число Т-хелперов (CD4+) снижено, а Т-цитотоксических клеток (CD8+) повышено [8]. Также показано, что у больных хроническим описторхозом, имевших высокую интенсивность инвазии, происходит достоверное снижение количества CD3+-, CD8+- и CD16+-клеток в периферической крови при одновременном повышении CD19+-B-лимфоцитов по сравнению с группой с низкой интенсивностью инвазии [9].
Отмечено, что в защите организма от паразитических инвазий участвуют макрофаги [10, 11]. Причём в одних случаях они активируются, что способствует борьбе организма с инвазией, в других подавляются, что в известной степени определяет дальнейшее развитие болезни, а иногда и неблагоприятный исход [12].
Особую роль в реализации противопаразитарного иммунитета при описторхозной инвазии играют эозинофилы [13]. Эозинофильные гранулоциты при гельминтозах выполняют различные функции, включая фагоцитоз многочисленных комплексов антиген-антитело, модуляцию гиперчувствительности и киллинг гельминтов при участии иммуноглобулинов G [14].
Вместе с тем остаётся открытым вопрос о влиянии метаболитов описторхисов на иммунный ответ в зависимости от длительности инвазии, так как информация об этих изменениях носит лишь экспериментальный характер.
Цель. Оценить клинические и иммунологические особенности у пациентов с хроническим описторхозом в зависимости от длительности инвазии.
Материал и методы исследования. В работе представлены материалы и результаты открытого проспективного исследования, проведённого в г. Екатеринбурге на базе инфекционного отделения №3 ГАУЗ СО «Городская клиническая больница №40». Первую группу составили 19 пациентов (7 мужчин и 12 женщин) с длительностью инвазии до 1 года в возрасте 42,4±1,73 года. Во вторую группу вошёл 21 пациент с длительностью инвазии от 1 до 5 лет (10 мужчин и 11 женщин) в возрасте 41,4±1,24 года. Третью группу сформировали 23 человека с длительностью инвазии более 5 лет (13 мужчин и 10 женщин) в возрасте 45,8±1,56 года.
Критерии включения:
1) мужчины и женщины в возрасте от 18 до 65 лет;
2) диагноз хронического описторхоза, подтверждённый обнаружением яиц Opisthorchis felineus в кале и/или жёлчи;
3) добровольное согласие пациентов на дегельминтизацию и лабораторное тестирование;
4) добровольное согласие больных на участие в клиническом исследовании;
5) место жительства пациентов — Свердловская область.
Критерии исключения:
1) сопутствующая другая паразитарная патология;
2) хроническое поражение печени, не обусловленное описторхозом (вирусные гепатиты, аутоиммунный гепатит, метаболические заболевания печени);
3) беременность и кормление ребёнка грудью;
4) острое инфекционное заболевание за 3 мес до начала исследования.
Проведение представленного исследования одобрено региональным этическим комитетом ФГБОУ ВО «Уральский государственный медицинский университет» Минздрава РФ, протокол №10 от 18.12.2015.
Диагноз хронического описторхоза у всех пациентов устанавливали на основании клинических, эпидемиологических, копроовоскопических и билиоовоскопических данных. Копроовоскопию проводили методом толстого мазка по Като с целлофаном и химико-седиментационным методом. У всех 63 пациентов обнаружены яйца сибирской двуустки.
Иммунологическое исследование проводили с письменного разрешения пациентов на базе МАУ «Клинико-диагностический центр». Параметры общего анализа крови регистрировались с помощью гематологического анализатора CobasMicros 60 (ABX). Иммунофенотипирование лимфоцитов осуществляли с использованием моноклональных антител CD3-FITC/CD20-PE, CD3-FITC/CD4-PE, CD3-FITC/CD8-PE, CD3-FITC/CD16+56-PE (IOTest) методом проточной цитофлюорометрии на цитометре FAC Scan (Becton Dickinson). Количество иммуноглобулинов классов М, G и А в сыворотке крови определяли методом радиальной иммунодиффузии в агаровом геле по G. Mancini. Содержание циркулирующих иммунных комплексов определяли методом преципитации их в 4% растворе ПЭГ-6000 по V. Haskova в модификации Ю.А. Гриневич. Результаты оценивали в единицах экстинции с помощью спектрофотометрии на аппарате СФ-46. Функционирование НАДФ-оксидазной1 системы нейтрофилов оценивали при помощи теста с нитросиним тетразолием (НСТ-теста).
Для оценки внутриклеточного киллинга (бактерицидной активности) лейкоцитов, завершённости фагоцитоза и оценки поглотительной активности нейтрофилов и моноцитов использовали метод, разработанный в лаборатории Института иммунологии МЗ РФ.
Иммунологическое исследование проводили до начала антигельминтной терапии. Основные критерии определения продолжительности инвазии: эпидемиологический анамнез (время употребления рыбы карповых пород), а также длительность клинической симптоматики. Все участники исследования употребляли в пищу рыбу карповых пород. Контрольную группу, сформированную методом случайной выборки, составили 20 соматически здоровых человек, у которых в течение жизни в пищевой рацион не входила рыба карповых пород и были отрицательными результаты кала на яйца гельминтов.
Статистическая обработка полученных данных проведена при помощи электронных программ Microsoft Excel 2010 и Statistica 6.0. Для сравнения полученных данных использовали методы непараметрического анализа с применением U-критерия Манна–Уитни. В том случае, если рассчитанное значение U-критерия было равно критическому или меньше, признавали статистическую значимость различий. Оценку интенсивности корреляционной связи (r) проводили с помощью рангового коэффициента корреляции Спирмена.
Результаты и обсуждение. Учитывая данные эпидемиологического анамнеза наших пациентов, удалось выявить следующие закономерности (табл. 1). В одинаковой степени в пищевой рацион входила только рыба карповых пород (вобла, карп, жерех, краснопёрка и др.): у больных при инвазии до 1 года (15,7%), от 1 года до 5 лет (14,2%), более 5 лет (17,3%). Морскую и рыбу карповых пород (в том числе вяленую) употребляли: при длительности инвазии до 1 года — 84,3%, от 1 года до 5 лет — 85,8%, более 5 лет — 82,6% пациентов. В рационе питания преимущественно рыба, выловленная из водоёмов Свердловской области, составляла у пациентов при инвазии до 1 года — в 84,2% случаев, от 1 года до 5 лет — в 90,4%, более 5 лет — в 73,2% случаев. Следует отметить, что наиболее часто ели рыбу, привозимую из водоёмов Тюменской области и Ханты-Мансийского автономного округа, в 17,4%, а Обь-Иртышского бассейна — в 8,7% случаев, пациенты с длительностью инвазии более 5 лет.
Таблица 1. Эпидемиологическая характеристика течения хронического описторхоза у пациентов в зависимости от длительности инвазии
Пищевой рацион | Инвазия до 1 года | Инвазия от 1 до 5 лет (n=21) | Инвазия более 5 лет | |||
абс. | % | абс. | % | абс. | % | |
Только рыба карповых пород | 3 | 15,7 | 3 | 14,2 | 4 | 17,3 |
Морская и рыба карповых пород (в том числе вяленая) | 16 | 84,3 | 18 | 85,8 | 19 | 82,6 |
Рыба из водоёмов Свердловской области | 16 | 84,2 | 19 | 90,4 | 17 | 73,9 |
Рыба из водоёмов Тюменской области и Ханты-Мансийского автономного округа | 2 | 10,5 | 1 | 4,8 | 4 | 17,4 |
Рыба из Обь-Иртышского бассейна | 1 | 5,3 | 1 | 4,8 | 2 | 8,7 |
Для постановки диагноза мы использовали классификацию Н.Н. Озерецковской и соавт. (1985) (табл. 2), согласно которой в клинической картине у наших пациентов выделялись следующие синдромы.
Таблица 2. Клиническая классификация описторхоза (по Н.Н. Озерецковской)
Фазы болезни | Клинический синдром | Органные поражения (клинический вариант течения) |
Острая | Субклинический. Основные: общие аллергические проявления, гепатохолангитический, гастроэнтеритический, тифоподобный. Редко встречающиеся: ангионевротический отёк гортани, синдром Лайелла, делирий и др. | Холангиохолецистит, гепатит, панкреатит, гастрит (катаральный, эрозивный), энтероколит, язва желудка и двенадцатиперстной кишки, астмоидный бронхит, пневмония, отёк мозга и др. |
Суперинвазия в острой форме | Аналогичные синдромам острой фазы | Аналогичные острой фазе |
Хроническая | Субклинический. Диспептический, астеноневротический, холецистокоронарный С.П. Боткина, панкреатокоронарный М.П. Кончаловского, лёгочный и др. | Холангиохолецистит, холангиогепатит, панкреатит, гастродуоденит, аллергический кожный, астмоидный бронхит, язва желудка и двенадцатиперстной кишки, стенозирующий папиллит и др. |
Суперинвазия в хронической фазе | Аналогичные синдромам острой фазы | Аналогичные поражениям острой фазы |
Реинвазия | Аналогичные синдромам острой фазы | Аналогичные поражениям острой фазы |
Резидуальный период острой фазы | Обратное развитие острой фазы болезни | Обратное развитие органных поражений острой фазы |
Резидуальный период хронической фазы | Компенсация или стабилизация хронической фазы | Клиническая компенсация или стабилизация органных поражений хронической фазы |
При инвазии до 1 года преобладало субклиническое течение (15,7%), отличительной особенностью которого были общая слабость, снижение работоспособности и нарушение сна. Следует отметить, что субклиническое течение при продолжительности заболевания от 1 до 5 лет встречалось у 9,5% человек, а при инвазии более 5 лет — в 8,7% случаев. Оно характеризовалось быстрой утомляемостью и раздражительностью. Этот вариант течения обусловлен тем, что в очагах описторхозной инвазии у коренного и местного населения могли складываться адаптационно-приспособительные отношения в системе защиты организма хозяина и в системах защиты и агрессии паразита, которые обеспечивают возможность их длительного сосуществования в условиях хронической инвазии [3, 15].
При инвазии от 1 до 5 лет доминировал холангиогепатит (57,1%), характеризовавшийся болью в правом подреберье (81,8%), непереносимостью острой и жареной пищи (41,6%), тошнотой (33,3%), кожным зудом (16,6%), гепатомегалией (25,0%). Реже этот синдром развивался при длительности инвазии до 1 года (31,5%), ведущим симптомом выступала тяжесть в правом подреберье (83,3%). Отличительной чертой холангиогепатита при инвазии более 5 лет (39,1%) были боли и тяжесть в правом подреберье (77,7%) и снижение аппетита (55,5%).
В патогенезе данного синдрома, по всей видимости, играло роль попадание восходящим путём описторхисов и их метаболитов, которые оказывали механическое и рефлекторное воздействие, а также вторично-инфекционный фактор, реализующийся нисходящим (гематогенным) путём, действующий на внутрипечёночные жёлчные протоки и печень [4, 16].
Аллергический кожный синдром превалировал при инвазии более 5 лет (47,8%), при длительности инвазии до 1 года его регистрировали в 26,3% случаев, а при инвазии от 1 года до 5 лет — у 28,5% пациентов. Он отличался волнообразным течением рецидивирующей крапивницы, наиболее значимыми симптомами которой были красные или бледно-розовые волдыри, преимущественно локализованные на животе, спине, верхних и нижних конечностях, в зоне декольте. Элементы сыпи были до 7–10 см в диаметре, а также сыпь распространялась на обширные участки тела. Зуд присутствовал на протяжении всего дня, часто вызывал бессонницу, а также периодический подъём температуры тела до субфебрильных цифр.
Аллергическая перестройка организма у пациентов возникала, судя по всему, в результате токсического и сенсибилизирующего воздействия продуктов обмена веществ и распада описторхисов, а также, возможно, аутосенсибилизации продуктами распада собственных тканей (клеток эпителия, выстилающего жёлчные протоки, подвергающихся некрозу при травматизации их гельминтами) [3, 5].
Холангиохолецистит также наиболее часто встречался при инвазии более 5 лет (65,2%). При инвазии до 1 года данный синдром встречался в 42,1% случаев, а при продолжительности заболевания от 1 года до 5 лет — у 38,1% больных. Основными симптомами холангиохолецистита выступали:
– при инвазии более 5 лет — давящие боли в правом подреберье, иррадиирующие в левую ключицу (53,3%), отрыжка (33,3%), изжога (26,6%), а также положительный симптом Ортнера (13,3%);
– при инвазии до 1 года — боли в правом подреберье, иррадиирующие в правую половину грудной клетки (37,5%), тошнота (62,5%) и субфебрильная температура тела (25,0%);
– при инвазии от 1 года до 5 лет — колющие боли в правом подреберье с иррадиацией в правую ключицу (37,5%), непереносимость жирной пищи (75,0%), а также положительный симптом Кера (12,5%).
В патогенезе холангиохолецистита большое значение отводят механическому и повреждающему действию паразитов на эпителиальные клетки гепатобилиарной системы. Своими присосками и шипиками они захватывают участки стенки протоков, скапливаются в них, создают препятствие для нормального оттока жёлчи и способствуют в последующем созданию благоприятных условий для присоединения вторичной бактериальной инфекции [2, 17, 18].
Патогенез хронического панкреатита складывался из попадания Op. felineus и его продуктов жизнедеятельности в протоки поджелудочной железы из двенадцатиперстной кишки через фатеров сосочек с дальнейшим повышением внутрипротокового давления и затруднением оттока сока и секрета, а также активацией вторичной микрофлоры и собственных ферментов [3, 15, 17, 18]. Отличительными клиническими признаками хронического панкреатита (30,4%) как ключевого варианта течения при инвазии более 5 лет были ноющие боли в околопупочной области (71,4%), иррадиирующие в правую лопатку (40,0%), вздутие живота (85,7%) и горечь во рту (57,1%). При длительности инвазии от 1 до 5 лет панкреатит развивался у 19,1% пациентов, характеризовался режущими болями в левом подреберье (25,0%), вздутием живота (75,0%) и метеоризмом (50,0%). При инвазии до 1 года поражение поджелудочной железы встречалось редко (10,5%), а клинические проявления сводились к опоясывающей боли в животе, кашицеобразному стулу и снижению аппетита (табл. 3). Следует отметить, что у наших больных при продолжительности инвазии более 5 лет наиболее продолжительно протекали рецидивы следующих вариантов течения: холангиохолецистита (3,08±1,02 мес), а также аллергического кожного синдрома (2,65±0,78 мес).
Таблица 3. Клиническая картина хронического описторхоза в зависимости от длительности инвазии
Клинический вариант течения хронического описторхоза | Инвазия до 1 года | Инвазия от 1 до 5 лет (n=21) | Инвазия более 5 лет | |||
абс. | % | абс. | % | абс. | % | |
Субклиническое течение | 3 | 15,7 | 2 | 9,5 | 2 | 8,7 |
Холангиогепатит | 6 | 31,5 | 12 | 57,1 | 9 | 39,1 |
Аллергический кожный синдром | 5 | 26,3 | 6 | 28,5 | 11 | 47,8 |
Холангиохолецистит | 8 | 42,1 | 8 | 38,1 | 15 | 65,2 |
Панкреатит | 2 | 10,5 | 4 | 19,1 | 7 | 30,4 |
Для выявления различных изменений иммунологической реактивности у наших пациентов трёх групп было проведено исследование иммунного статуса.
В процессе работы удалось выявить, что развитие хронического иммуновоспалительного процесса при описторхозе в зависимости от продолжительности заболевания имело следующие особенности (табл. 4): увеличение уровня лимфоцитов, клеток, образующих первую линию защиты от гельминтов, зарегистрировано при инвазии до 1 года (2,74±0,33; р=0,021) и более 5 лет (2,66±0,54; р=0,032) в сравнении с группой контроля (2,41±0,18; р=0,042).
Таблица 4. Иммунологические показатели у больных хроническим описторхозом в зависимости от длительности инвазии
Показатель | Группа больных, M±m | Контрольная группа (n=20) | ||
Первая группа: инвазия до 1 года (n=19) | Вторая группа: инвазия от 1 до 5 лет (n=21) | Третья группа: инвазия более 5 лет (n=23) | ||
Лимфоциты, ×109/л | 2,74±0,33* | 1,81±0,32 | 2,66±0,54* | 2,41±0,18 |
Моноциты, ×109/л | 0,76±0,05 | 0,89±0,06* | 0,64±0,05 | 0,58±0,04 |
Гранулоциты, ×109/л | 3,26±1,22 | 4,12±1,65 | 2,74±0,37 | 4,21±0,98 |
Эозинофилы, ×109/л | 0,16±0,02 | 0,18±0,03 | 0,34±0,03*/*** | 0,29±0,02 |
Скорость оседания эритроцитов, мм/ч | 12,6±0,98 | 14,1±1,23 | 11,7±1,12 | 12,5±1,71 |
Иммуноглобулины M, г/л | 1,74±0,32* | 1,66±0,43* | 1,68±0,48* | 1,24±0,29 |
Иммуноглобулины G, г/л | 13,6±1,38 | 18,4±1,76* | 20,6±1,95* | 12,9±1,09 |
Циркулирующие иммунные комплексы, ед. | 106,2±2,61*/*** | 78,4±1,96* | 58,9±1,88 | 54,2±3,11 |
CD19+ | 0,17±0,02 | 0,26±0,03 | 0,34±0,04 | 0,27±0,02 |
CD3+ | 0,97±0,04* | 1,36±0,05 | 0,89±0,05* | 1,24±0,13 |
CD4+ | 0,59±0,03 | 0,58±0,02 | 0,56±0,02 | 0,76±0,19 |
CD8+ | 0,56±0,02 | 0,45±0,03* | 0,76±0,03 | 0,59±0,11 |
NK-клетки | 0,26±0,01 | 0,49±0,03*/** | 0,36±0,02* | 0,28±0,04 |
Стимулированный тест с нитросиним тетразолием, % | 26,1±1,39 | 12,7±1,01*/** | 14,4±1,48* | 28,1±2,78 |
Бактерицидная активность лейкоцитов, % | 63,2±2,84*/** | 34,9±2,32 | 42,1±2,66 | 46,7±3,34 |
Поглотительная активность моноцитов, % | 69,1±2,04 | 82,4±2,81* | 79,4±2,11* | 57,4±3,97 |
Поглотительная активность нейтрофилов, % | 89,4±2,96 | 87,2±3,03 | 92,4±3,07 | 83,1±4,59 |
Примечание: уровень достоверности различий р <0,05: *при сопоставлении с группой контроля; **при сравнении длительности инвазии до 1 года с длительностью инвазии от 1 до 5 лет; ***при сравнении длительности инвазии до 1 года и длительности инвазии более 5 лет (непараметрический метод Манна–Уитни — U-критерий).
Во второй группе показатели лимфоцитов находились в пределах референтных значений (1,81±0,32; р=0,081). Зарегистрировано повышение количества моноцитов (0,89±0,06; р=0,047) — клеток, ответственных за неспецифическую защиту организма, а также, играющих огромную роль в процессе воспаления и противопаразитарной защите, в сопоставлении с показателями здоровых добровольцев (0,58±0,04; р=0,038). Показатели моноцитов в первой (0,76±0,05; р=0,074) и третьей (0,64±0,05; р=0,092) группах не выходили за пределы нормы.
Наиболее высокое количество эозинофилов, которые, скорее всего, прикреплялись к гельминтам, локально высвобождали содержимое гранул и повреждали оболочку паразита, а также участвовали в воспалительных реакциях [13, 14, 19], обнаружено при инвазии более 5 лет (0,34±0,03; р=0,046) в сопоставлении с первой группой (0,16±0,02; р=0,036) и уровнем здоровых людей (0,29±0,02; р=0,084).
Изменение в гуморальном звене в виде увеличения уровня иммуноглобулинов M выявлено во всех группах: при инвазии до 1 года (1,74±0,32; р=0,033), от 1 до 5 лет (1,66±0,43; р=0,038) и более 5 лет (1,68±0,48; р=0,043) в сравнении с контрольной группой (1,24±0,29; р=0,044). Наиболее высокая концентрация циркулирующих иммунных комплексов, которые отражают в первую очередь антигельминтную нагрузку, выявлена при инвазии до 1 года (106,2±2,61; р=0,041) в сопоставлении с третьей группой (58,9±1,88; р=0,065) и группой контроля (54,2±3,11; р=0,088). Усиление синтеза иммуноглобулинов G, которое свидетельствовало о длительном активном хроническом аллергическом воспалении и участии его во вторичном иммунном ответе, зарегистрировано при длительности инвазии от 1 до 5 лет (18,4±1,76; р=0,029) и более 5 лет (20,6±1,95; р=0,024) в сравнении с контрольной группой (12,9±1,09; р=0,025).
В первой (0,97±0,04; р=0,027) и третьей (0,89±0,05; р=0,039) группах в клеточном звене иммунитета выявлено снижение количества CD3+-лимфоцитов по сравнению со значениями здоровых добровольцев (1,24±0,13; р=0,045), которое можно рассматривать как реакцию иммунитета на внедрение во внутреннюю среду патогена. Это характеризовало начало формирования ответной защитной реакции [8, 20]. Между тем, при длительности инвазии от 1 до 5 лет зафиксирован дисбаланс в клеточном звене иммунитета со снижением содержания CD8+-лимфоцитов (0,45±0,03; р=0,042) в сравнении с контрольной группой (0,59±0,11; р=0,076), а также наиболее высокие показатели NK-клеток (0,49±0,03; р=0,041) в сопоставлении с первой группой (0,26±0,01; р=0,034) и здоровыми людьми (0,28±0,04; р=0,055), свидетельствовавшие о компенсаторно-приспособительной реакции Т-клеток в условиях длительной антигенной стимуляции Opisthorchis felineus [8, 21, 22].
Среди неспецифических механизмов защиты при длительности инвазии до 1 года происходил наиболее высокий рост бактерицидной активности лейкоцитов (63,2±2,84; р=0,021) по сравнению со второй группой (34,9±2,32; р=0,041) и группой контроля (46,7±3,34; р=0,062), приводящий к готовому потенциалу со способностью фагоцитарного звена иммунитета стать одним из главных участников ответных иммуновоспалительных реакций, происходящих в тканях макроорганизма [10, 11, 23].
Следует отметить, что в фагоцитарном звене иммунитета во второй группе (12,7±1,01; р=0,033) в сравнении со значениями первой группы (26,1±1,39; р=0,038) и здоровых людей (28,1±2,78; р=0,054) выявлены наиболее низкие показатели НСТ-теста, вероятно, связанные с ослаблением поглотительной функции фагоцитов, которые восполнялись поглотительной активностью моноцитов (82,4±2,81; р=0,047) в сопоставлении с показателями контрольной группы (57,4±3,97; р=0,065) [20, 23]. В свою очередь, при длительности инвазии более 5 лет на фоне снижения НСТ-теста (14,4±1,48; р=0,045) в сравнении с группой контроля и активации поглотительной активности моноцитов (79,4±2,11; р=0,048) в сопоставлении со здоровыми добровольцами, выявлено увеличение количества NK-клеток (0,36±0,02; р=0,047) в сопоставлении со здоровыми людьми, что могло свидетельствовать о взаимной адаптации паразита и Т-клеточного звена иммунитета на длительный срок [8, 20, 22].
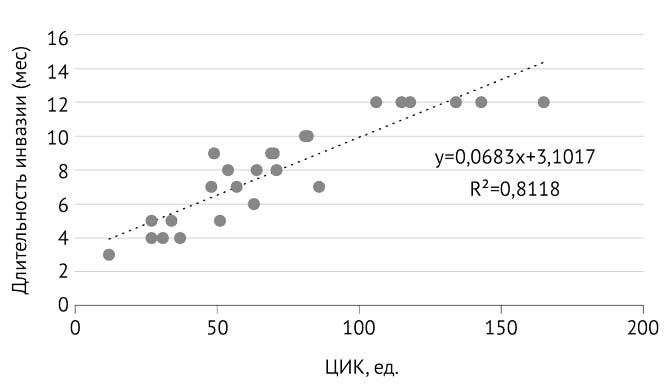
В дальнейшем мы оценили зависимость между длительностью описторхозной инвазии и иммунологическими показателями (табл. 5). Установлена содружественная реакция между продолжительностью инвазии и количеством циркулирующих иммунных комплексов: до 1 года присутствовала сильная связь (r=+0,811; р=0,022), а от 1 до 5 лет (r=+0,284; р=0,036) и более 5 лет (r=+0,161; р=0,042) — прямая связь слабой силы. Увеличение концентрации иммунных комплексов способствовало гуморальной иммунной защите от антигенов гельминта, которые они связывали и обезвреживали с последующим их уничтожением/удалением из организма [24, 25] (рис. 1).
Таблица 5. Корреляция между длительностью инвазии и иммунологическими показателями у больных хроническим описторхозом (р <0,05).
Клиническая группа | Показатель | ||||
Циркулирующие иммунные комплексы | CD3+ | Иммуноглобулины G | CD4+ | CD8+ | |
Хронический описторхоз с длительностью инвазии до 1 года (n=19) | +0,811 | –0,028 | +0,024 | –0,34 | +0,213 |
Хронический описторхоз с длительностью инвазии от 1 до 5 лет (n=21) | +0,284 | +0,781 | +0,845 | +0,038 | –0,227 |
Хронический описторхоз с длительностью инвазии более 5 лет (n=23) | +0,161 | –0,022 | +0,281 | +0,797 | +0,835 |
Примечание: r=0–0,3 — слабая связь; r=0,3–0,7 — связь средней силы; r=0,7–1 — сильная связь; в случае результата со знаком «+» — связь прямая, со знаком «–» — связь обратная.
Рис. 1. Корреляционная связь между длительностью инвазии до 1 года и уровнем циркулирующих иммунных комплексов (ЦИК)
Кроме того, отмечен антагонизм длительности инвазии до 1 года (r=–0,028; р=0,042) и более 5 лет (r=–0,022; р=0,032), а также сильная положительная связь длительности от 1 до 5 лет (r=+0,781; р=0,034) с CD3+-лимфоцитами. Повышение содержания CD3+-лимфоцитов, скорее всего, свидетельствовало о гиперактивности иммунитета, а на фоне полиморфизма клинических проявлений указывало на вялое течение хронического воспалительного процесса [8, 26] (рис. 2).
Рис. 2. Корреляционная связь между длительностью инвазии от 1 до 5 лет и уровнем CD3+-лимфоцитов
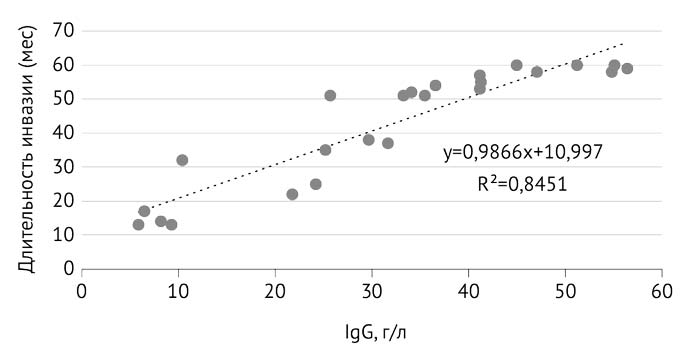
Вне зависимости от продолжительности инвазии зафиксирован синергизм с иммуноглобулином G: до 1 года (r=+0,024; р=0,046), от 1 до 5 лет (r=+0,845; р=0,041), более 5 лет (r=+0,281; р=0,044). Продукты жизнедеятельности Opisthorchis felineus, по всей видимости, в процессе хронизации приводили к иммуносупрессии и становились мощным триггером для синтеза иммуноглобулина G, который участвовал во вторичном иммунном ответе [25, 27] (рис. 3).
Рис. 3. Корреляционная связь между длительностью инвазии от 1 до 5 лет и уровнем иммуноглобулина G (IgG)
В свою очередь, выявлена отрицательная связь средней силы между длительностью инвазии до 1 года (r=–0,34; р=0,041) в противовес прямой зависимости от 1 до 5 лет (r=+0,038; р=0,026), а также более 5 лет (r=+0,797; р=0,045) и Т-хелперов, которая свидетельствует о том, что CD4+ активно участвовали в формировании адаптивного иммунного ответа, воспринимали продукты метаболизма кошачьей двуустки как «раздражитель» и в последующем, возможно, передавали сигналы B-клеткам и Т-эффекторам [22, 28] (рис. 4).
Рис. 4. Корреляционная связь между длительностью описторхозной инвазии и уровнем Т-хелперов
Следует отметить, отрицательную связь слабой силы между продолжительностью инвазии от 1 до 5 лет (r=–0,027; р=0,034) и Т-цитотоксических лимфоцитов. Увеличение продукции CD8+-лимфоцитов у пациентов с инвазией, протекающей до 1 года (r=+0,213; р=0,037) и более 5 лет (r=+0,835; р=0,035), возможно, обусловлено клеточной реакцией при более длительной антигенной стимуляции [8, 25, 29] (рис. 5).
Рис. 5. Корреляционная связь между длительностью описторхозной инвазии и уровнем цитотоксических лимфоцитов
Следовательно, в хронической фазе описторхозной инвазии напряжённость фагоцитарного, клеточного и гуморального звеньев иммунитета у наблюдаемых больных зависела от длительности заболевания и эозинофильной реакции.
Выводы
- У больных хроническим описторхозом преобладала классическая клиническая картина со следующими вариантами течения: холангиохолецистит, холангиогепатит, панкреатит. Особенность инвазии, длящейся более 5 лет, — частый и продолжительный рецидив аллергического кожного синдрома (2,65±0,78 мес).
- Иммунный ответ при хроническом описторхозе характеризовался: (1) при инвазии до 1 года — лимфоцитозом, повышением уровня иммуноглобулина M и циркулирующих иммунных комплексов, снижением количества CD3+-лимфоцитов, а также увеличением бактерицидной активности лейкоцитов; (2) от 1 до 5 лет — моноцитозом, повышением содержания иммуноглобулинов M, G и циркулирующих иммунных комплексов, снижением содержания CD8+-лимфоцитов и результатов теста с нитросиним тетразолием, а также увеличением количества NK-клеток и активацией поглотительной активности моноцитов; (3) более 5 лет — эозинофилией.
- Общие черты иммунологической перестройки при описторхозной инвазии: воспалительные изменения в гемограмме, активация гуморального с параллельным угнетением клеточного звена иммунитета, а также усиление неспецифических механизмов защиты.
Участие авторов. Д.А.С. проводил исследование и анализировал результаты; Ю.Б.Х. отвечала за сбор информации; А.У.С. — руководитель работы.
Источник финансирования. Исследование не имело спонсорской поддержки.
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов по представленной статье.
About the authors
A U Sabitov
Ural State Medical University
Email: soldatoff.mitya2012@yandex.ru
Russian Federation, Yekaterinburg, Russia
D A Soldatov
Ural State Medical University
Author for correspondence.
Email: soldatoff.mitya2012@yandex.ru
Russian Federation, Yekaterinburg, Russia
Yu B Khamanova
Ural State Medical University
Email: soldatoff.mitya2012@yandex.ru
Russian Federation, Yekaterinburg, Russia
References
- Beloborodova E.I., Svyatenko I.A., Beloborodova E.V. Course of gastroesophageal reflux disease on a background of chronic opisthorchiasis. Klinicheskie perspektivy gastroenterologii, gepatologii. 2011; (4): 26–30. (In Russ.)
- Krivosheev A.B., Khvan L.A. Chronic opisthorchiasis and biliary dysfunction. Poliklinika. 2017; (3): 28–30. (In Russ.)
- Kuznetsova V.G., Krasnova E.I., Paturina N.G. Opisthorchiasis in the clinical practice of an infectious disease doctor. Attending physician. 2013; (6): 74–78. (In Russ.)
- Paltsev A.I., Eremina A.A. Non-alcoholic fatty liver disease and opisthorchiasis. Features of the clinic, diagnosis, treatment. Doktor.Ru. 2015; (2-2): 57–58. (In Russ.)
- Drynov G.I., Ushakov D.V. Parasitic allergy. Infectious diseases: news, opinions, training. 2014; (1): 28–32. (In Russ.)
- Peckruhn M., Elsner P., Tittelbach J. Eosinophilic dermatoses. J. Dtsch. Dermatol. Ges. 2019; 17 (10): 1039–1051. doi: 10.1111/ddg.13943.
- Grishina E.A. The role of cytokines in the immunity development at helminthiasis. Russian Journal of Parasitology. 2016; 38 (4): 521–526. (In Russ.) doi: 10.12737/23077.
- Kawraemruaen C., Sermswan R.W., Wongratanacheewin S. Induction of regulatory T cells by Opisthorchis viverrini. Parasite Immunol. 2016; 38 (11): 688–697. doi: 10.1111/pim.12358.
- Ilyinskikh E.N., Ilyinskikh I.N., Ilyinskikh N.N., Lepyokhin A.V., Yurkin A.Yu., Buzhak N.S. Characterization of cellular immune response associated with infection intensity in chronic opisthorchiasis patients. Bulletin of Siberian Medicine. 2010; (1): 40–44. (In Russ.)
- Bai X., Yu J.L., Wang F., Zhao Y., Liu M.Y., Wang G.M. Alternatively activated macrophages in helminth infections. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing ZaZhi. 2011; 29 (3): 219–23. (In Chinese.) PMID: 21970115.
- Salao K., Watakulsin K., Mairiang E., Suttiprara S., Tangkawattana S., Edwards S.W., Sripa B. High macrophage activities are associated with advanced periductal fibrosis in chronic Opisthorchis viverrini infections. Parasite Immunol. 2019; 41 (1): e12603. doi: 10.1111/pim.12603.
- Jenkins S.J., Allen J.E. Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes. J. Biomed. Biotechnol. 2010; 2010: 262609. doi: 10.1155/2010/262609.
- Bychkov V.G., Pankov I.V., Chernov I.A., Kulikova S.V., Lazarev S.D. Sudden cardiac death in hypereosinophilic syndrome against the background of superinvasive opisthorchiasis. Forensic medicine. 2019; 5 (S1): 97–98. (In Russ.)
- Chernogoryuk G.E., Roslyakova E.P., Shepeleva E.G., Denisova O.A., Mikhaylova A.A., Tyukalova L.I., Varvyanskaya N.V., Rachkovskiy M.I., Kalyuzhina E.V., Garganeeva N.P. Eosinophils and bronchial obstruction in patients with copd and chronic opisthorchis felineus infection. Modern problems of science and education. 2016; (6): 270. (In Russ.)
- Pozdnyakova L.L., Krasnova E.I., Kuznetsova V.G., Malov I.V. Opistorkhoz u vzroslykh. Klinicheskie rekomendatsii. (Opisthorchiasis in adults. Clinical guidelines.) Novosibirsk. 2014; 53 p. (In Russ.)
- Dietrich C.F., Atkinson N.S.S., Lee W.J., King K., Neumayr A., Braden B., Richter J., Akpata R., Southisavath P., Schreiber-Dietrich D., Dong Y. Never seen before? Opisthorchiasis and clonorchiasis. Z. Gastroenterol. 2018; 56 (12): 1513–1520. doi: 10.1055/а-0751-3078.
- Plotnikova E.Yu., Baranova E.N. Opistorchoz: complications and problems of treatment. Gastroenterologiya Sankt-Peterburga. 2018; (3): 14–18. (In Russ.)
- Matveeva M.Yu., Ofitserov V.I. Mediko-biologicheskie osobennosti opistorkhoza. Informatsionno-metodicheskoe posobie. (Biomedical features of opisthorchiasis. Informational and methodological guide.) Novosibirsk: Vector-Best. 2018; 32 p. (In Russ.)
- Huang L., Appleton J.A. Eosinophils in helminth infection: Defenders and dupes. Trends Parasitol. 2016; 32 (10): 798–807. doi: 10.1016/j.pt.2016.05.004.
- Motran C.C., Silvane L., Chiapello L.S., Theumer M.G., Ambrosio L.F., Volpini X., Celias D.P., Cervi L. Helminth infections: Recognition and modulation of the immune response by innate immune cells. Front. Immunol. 2018; 9: 664. doi: 10.3389/fimmu.2018.00664.
- Maizels R.M. Regulation of immunity and allergy by helminth parasites. Allergy. 2020; 75 (3): 524–534. doi: 10.1111/all.13944.
- Knipper J.A., Ivens A., Taylor M.D. Helminth-induced Th2 cell dysfunction is distinct from exhaustion and is maintained in the absence of antigen. PLos Negl. Trop. Dis. 2019; 13 (12): e0007908. doi: 10.1371/journal.pntd.0007908.
- Coakley G., Harris N.L. Interactions between macrophages and helminthes. Parasite Immunol. 2020; 42 (7): e12717. doi: 10.1111/pim.12717.
- Haase P., Voehringer D. Regulation of humoral type 2 immune response against allergens and helminthes. Eur. J. Immunol. 2021; 51 (2): 273–279. doi: 10.1002/eji.202048864.
- Sripa B., Jumnainsong A., Tangkawattana S., Haswell M.R. Immune response to Opisthorchis viverrini infection and its role in pathology. Adv. Parasitol. 2018; 102: 73–95. doi: 10.1016/bs.apar.2018.08.003.
- Logan J., Navarro S., Loukas A., Giacomin P. Helminth-induced regulatory T cells and suppression of allergic responses. Curr. Opin. Immunol. 2018; 54: 1–6. doi: 10.1016/j.coi.2018.05.007.
- Harris N., Gause W.C. To B or not to B: B cells and the Th2-type immune response to helminthes. Trends Immunol. 2011; 32 (2): 80–88. doi: 10.1016/j.it.2010.11.005.
- Gazzinelli-Guimaraes P.H., Nutman T.B. Helminth parasites and immune regulation. F1000Res. 2018; 7 (F1000 Faculty Rev): 1685. doi: 10.12688/f1000research.15596.1.
- Douglas B., Oyesola O., Cooper M.M., Posey A., Tait Wojno E., Giacomin P.R., Herbert D.R. Immune system investigation using parasitic helminths. Annu. Rev. Immunol. 2021; 39: 639–665. doi: 10.1146/annurev-immunol-093019-122827.
Supplementary files